A year has passed and the world is still at war with the coronavirus pandemic. However, towards the end of 2020 – on 10 November to be exact – an announcement by Pfizer regarding its coronavirus vaccine raised hopes of an end to the worst pandemic in a century. Today, a number of vaccines have been approved for emergency use in various countries.
Nevertheless, the COVID-19 pandemic is “far from over,” said Tedros Adhnom, Director-General of the World Health Organization (WHO). At the time of writing, over 96 million people worldwide have been affected by the deadly disease with over two million COVID-related deaths reported.
In recent weeks, many countries have been reporting record highs, new waves, and imposed fresh lockdowns and preventive measures on its citizens.
Therefore, it is not a surprise that governments are scrambling to purchase vaccines and getting them approved as soon as they can. The United Kingdom (UK) became the first country to authorise a COVID-19 vaccine – which was then followed by Belgium, Canada, Israel, and Singapore, among others. The latter began its coronavirus vaccination campaign in late December – making it the first Southeast Asian country to roll out inoculations.
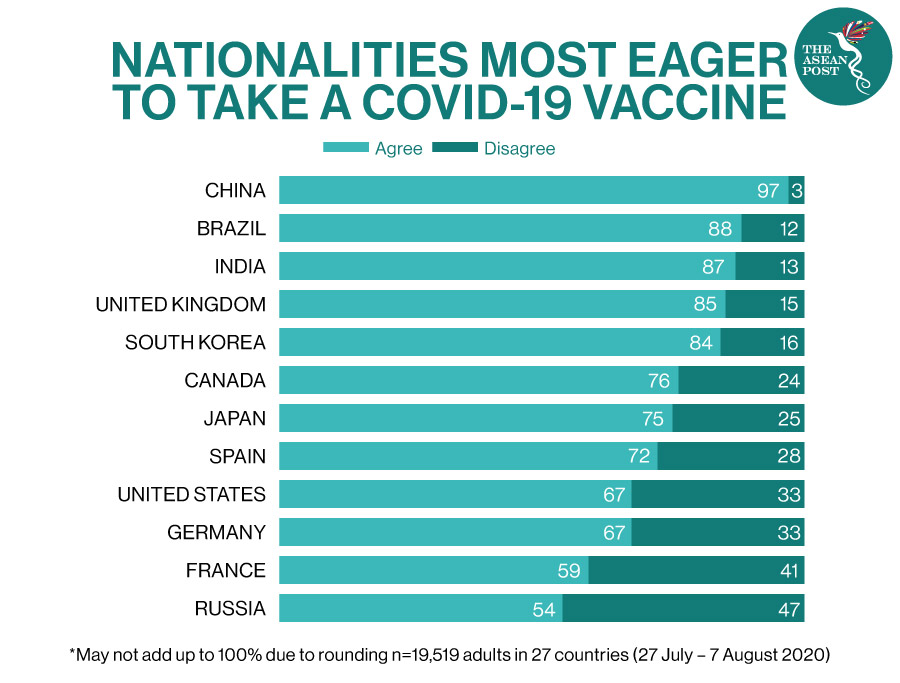
While vaccines from manufacturers like Pfizer, Moderna, Russia’s Gamaleya (Sputnik V), and AstraZeneca have been making headlines with their respective rollouts and effectiveness – others like China’s Sinovac has been widely discussed more than others in recent days.
China’s Sinovac vaccine has been found to be 50.4 percent effective in Brazilian clinical trials – significantly lower than initial results had shown. Researchers at the Butantan Institute, which has been conducting trials in Brazil, has earlier announced that the vaccine had a 78 percent efficacy against “mild-to severe” cases.
But last week, they revealed that calculations for this figure did not include data from a group of “very mild infections” among those who received the vaccine that did not require clinical assistance. With the inclusion of this data, the efficacy rate is now just 50.4 percent, said the researchers.
This has raised a growing number of questions over the effectiveness of the Sinovac vaccine despite rollouts already happening in some countries.
CoronaVac
The aforementioned vaccine – the CoronaVac – developed by Beijing-based biopharmaceutical company Sinovac is based on traditional technology that uses inactivated or a dead virus, that cannot replicate in human cells, to trigger an immune response.
One of the main advantages of the vaccine is that it can be stored in a standard refrigerator at two to eight degrees Celsius (C) compared to Moderna’s vaccine which needs to be stored at -20 C and Pfizer’s vaccine at -70 C. This means that the Sinovac vaccine is a lot more practical for deployment in developing countries that might not be able to store large amounts of vaccines at very low temperatures for a prolonged period.
Despite some Western media questioning its safety, more countries have announced that they would purchase CoronaVac or start mass inoculations using them. It was reported that Turkey has started administering the vaccine to its healthcare staff and is expected to vaccinate all healthcare professionals by next week. The country has agreed to purchase 50 million doses of the Sinovac vaccine and has already received delivery of an initial three million doses.
Closer to home, Indonesian President Joko Widodo received the first dose of CoronaVac last week which was aired live on television. In an interview with Global Times, Djauhari Oratmangun, the Indonesian Ambassador to China said that the archipelago had already begun mass vaccinations, thanks to its good collaboration with China.
"Indonesia's trust and confidence in Sinovac's vaccine is reflected in Widodo being the first to receive a shot. We share President Widodo's sentiments," the ambassador said.
Philippine President Rodrigo Duterte also praised the Chinese-made vaccine, saying that “the Chinese are bright. They would not venture (into producing vaccines) if it is not safe, sure and secure.” This was in reference to doubts about the level of protection Sinovac’s vaccine can provide.
However, following the release of the aforementioned Brazilian trial data, some countries are re-examining potential purchase plans. For instance, Malaysia said that it would seek more data from Sinovac before it approved a purchase. Whereas in Hong Kong, which has signed a deal with the Chinese firm, a senior medical adviser said an expert panel would review every vaccine based on clinical trial data.
‘Good Enough’
Part of the confusion over Sinovac’s vaccine has been around different efficacy rates and the data made available. Turkey and Indonesia both reported efficacy rates of 91.25 percent and 65.3 percent, respectively. Whereas as mentioned earlier, the Butantan Institute revealed a 50.4 percent efficacy rate.
In response to the Brazilian clinical trials, Sinovac Biotech has defended its vaccine. They said that the participants in its Phase III trial in Brazil – the largest one for the vaccine undertaken worldwide – were medical workers who tended to COVID-19 patients, arguing that they faced higher exposure to the highly infectious pathogen.
"The vaccine's ability to protect medical workers in active outbreaks could significantly improve if they are administered between 21 and 28 days," said the company in a statement.
Experts also agreed that the overall result is good enough considering almost all the trial participants in Brazil were high-risk front-liners, as reported in the Global Times.
Moreover, the Butantan Institute stressed that the vaccine is 78 percent effective in preventing mild cases that needed treatment and 100 percent effective in starving off moderate to serious cases.
Additionally, 50.4 percent – meets the 50 percent minimum threshold required by most regulators around the world, but is lower than the 70 percent recommended by the WHO.
Related Articles: