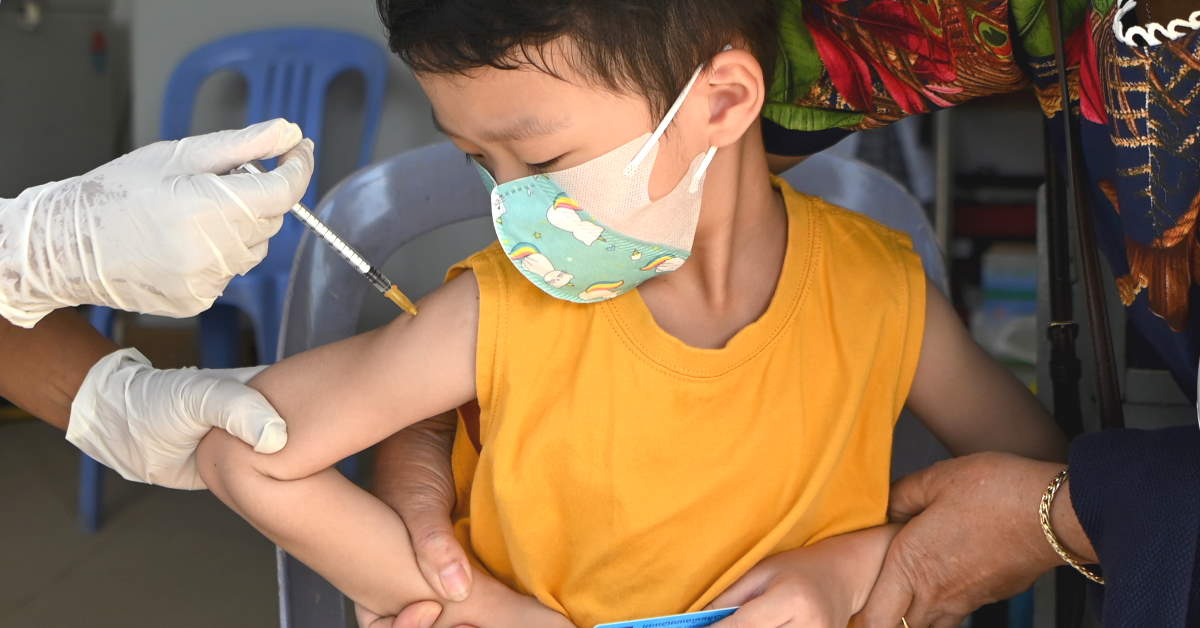
Children under the age of five are not yet able to be vaccinated against COVID leaving some parents worried their younger children could catch the virus from older siblings who have returned to school.
Less than 50 percent of children aged five to 11 have received their first vaccine dose. But it is hoped child vaccination rates will be bolstered by Australia becoming the first country to approve the Moderna vaccine for children aged six and over. Pfizer continues to be available to children aged five and over.
But will children under five ever be eligible for a COVID-19 vaccine? How many doses will be enough for children? And is a first-dose rate of less than 50 percent in the five to 11-year-old age group enough to protect the most vulnerable?
Will COVID Vaccines Ever Be Available For Under-Fives?
Australia’s drugs regulator, the Therapeutic Goods Administration (TGA), has not yet received an application from any pharmaceutical company to extend the use of any approved COVID vaccine to children under five.
If an application is made, it will need to include data from clinical trials to demonstrate the vaccine is safe and effective in this age group. A spokesperson from the TGA said any such application “will be evaluated by the TGA with the greatest priority”. Pfizer has clinical studies under way in the United States (US) of its COVID vaccine in children aged from six months to four years old, with results due in April.
The Pfizer dosage for anyone aged 12 and above is 30 micrograms a dose, while children aged five to 12 receive 10 micrograms a dose. In the Pfizer trial for children under five, two doses of three micrograms were given.
In December, Pfizer said it was changing the design of the trial to include a third dose. This was because while the three micrograms dosage for six-month to two-year-olds produced an immune response as effective as that seen in children aged five and up, the same dosage generated an inferior immune response in the two to five-year-old age group.
If a vaccine for children under five is eventually registered by the TGA, the health department will seek final advice and approval from its independent panel of vaccination experts, Atagi. This is an important step because Atagi will carefully consider the relevant risks, benefits and uncertainties of the evidence, from the clinical trials and any real-world use of the vaccine in this age group that may become available over time.
Why Do Countries Differ In Their Vaccination Advice For Children?
In Australia, the vaccination rollout for children aged five to 11 started on 10 January. But in England, children in the same age group will not be offered the COVID vaccine until April. For now, England is focusing its vaccination efforts on immunocompromised children aged five to 11 and adolescents.
The rollout for children in England is being described as “non-urgent” by health officials compared with its rollout for older children and adults. In Australia, increasing the vaccine uptake in the five to 11-year-old group is being treated with more urgency.
The US has been pushing for vaccination of five to 11-year-olds since October. More than seven million children in the age group have safely received doses of the Pfizer vaccine in the US. There are unique contexts in each country that regulators consider, including what proportion of the population is vaccinated or has already had COVID, the variants circulating and the proportion of vulnerable people vaccinated or at risk.
The World Health Organization (WHO) advises countries to consider the individual and population-level benefits of immunising children and adolescents according to their own epidemiological and social contexts.
The UK’s Joint Committee on Vaccination and Immunisation (JCVI) had previously taken the view that it may be unethical to vaccinate children in order to protect others, given children do not normally suffer serious symptoms from COVID-19, but can very rarely experience serious but treatable vaccine side-effects.
JCVI previously said that although the health gains from vaccinating children are greater than the risks, “the margin of benefit is considered too small” to support broad child vaccination on health grounds alone. This advice changed in February when JCVI recommended non-urgent vaccination of children to protect against rare cases of severe illness, in advance of a potential future coronavirus wave. However, the experts emphasised that vaccination of the five to 11 age group may not be recommended in the future.
In Australia, regulators took the view that vaccinating children would probably directly benefit all in the community through decreased infection and severe disease, while also having indirect benefits through reduced transmission and reduced disruption to schooling, sports and other organised activities.
Will Australian Regulators Approve A Booster Shot For Children Under 16 Years Old?
In Australia, those aged five and over who have an immunocompromised condition are already advised to receive a third dose.
The TGA has not received an application to extend the use of any COVID-19 vaccine as a booster for those aged under 16 more generally. But the Royal Australian College of Physicians president-elect and paediatrician, Dr Jacqueline Small, said the two-dose vaccination course generates a strong immune response in young people.
“It is too early to tell how long immunity will last in children aged under 16 years and what benefit booster doses would provide,” Small said. “However, there is some suggestion [two doses] may be longer lasting than in adults. It is vitally important that the current focus on COVID vaccination not displace existing vaccinations as recommended in National Immunisation Program Schedule. There are growing concerns that other health care interventions, including other childhood vaccinations, are being neglected through the pandemic.
“Data also shows that severe disease or death was significantly lower in adolescents who had received two doses of vaccine compared those who were unvaccinated.”
Should I Be Worried If My Child Is Not Old Enough To Be Vaccinated?
Small said the best protection for children under five, until a vaccine is available, is for adults and older children to be vaccinated. Other public health measures, including hand hygiene, social distancing, cough and sneeze etiquette and mask wearing also help prevent infection during significant community transmission.
“It is important that children attend school and participate in other activities that are important for their health and development,” she said.
“We know that COVID-19 infection in children generally involves no symptoms or causes a brief illness with mild symptoms.”
My Child Has Had COVID, What Is The Benefit Of Vaccinating Them As Well?
Fiona Russell, a paediatrician and professor at the University of Melbourne, said even if a child has already been infected, vaccination is recommended if they are eligible.
“Recent data has shown evidence of prior infection recently jumped from 55 percent to 97 percent in eight to 11-year-olds in the UK within a few weeks during the Omicron outbreak, indicating just how fast and transmissible this variant is,” she said.
While hospitalisations from Omicron are rare, they do occur in about three in 100,000 children, according to data from the Netherlands and US during their virus peaks.
“About one in 3,000 go on to develop a treatable multi-system inflammatory condition after the acute infection,” Russell said.
“Reinfections occur. These things are vaccine-preventable, so vaccination is recommended.
“No parent would want their child to be a statistic.”